Batteries
Batteries are widely used across campus, and come in multiple forms. Rechargeable lithium-ion (Li-ion) and lithium polymer (LiPo) are commonly used in consumer electronics (cell phones, tablets, laptops, power tools, electronic cigarettes, etc.). Lead-acid batteries are used in automobiles; alkaline, lithium, silver oxide, nickel-cadmium (NiCd), nickel-metal hydride (NiMH) and many other types of batteries exist. Many types of batteries can be used to power the same equipment, but their safety and disposal considerations can vary. Recognizing the differences and the hazards associated with them is crucial when working with batteries. For example, lithium-ion and lithium batteries are very different. Developing new and improving existing batteries is an active area of study. Research on battery technology is active on campus.
Battery Basics
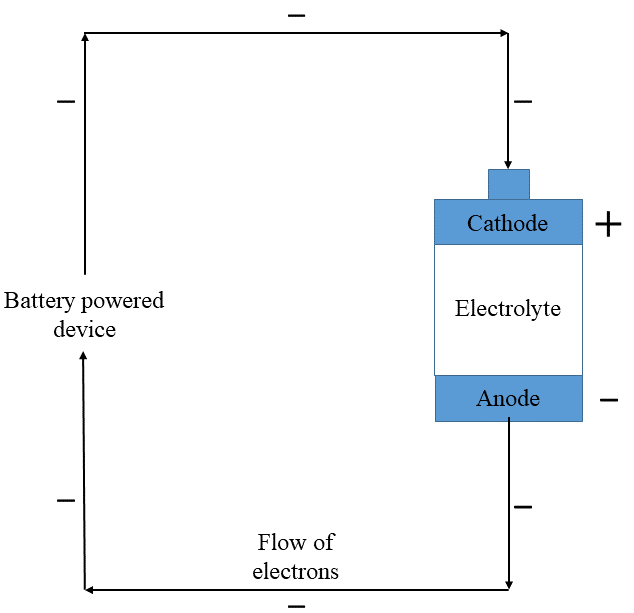
Batteries are portable devices containing electrochemical cells that power electrical equipment. Batteries consist of a positively charged cathode, a negatively charged anode, and a conductive electrolyte containing anions and cations. These are separated into two half-cells that make up the battery. One half-cell contains a negative electrode and electrolyte. The second includes the positive electrode and electrolyte. Together, a redox reaction occurs at opposite ends of the cell, allowing electrons to flow out of the negative end and into the electrical device, while the electrolyte carries charge between the electrodes.
The chemicals used in the anode, cathode, and electrolyte vary, which creates the wide variety of batteries available along with a wide variety of hazards.
Primary And Secondary Batteries
Batteries are classified as primary or secondary.
- Primary batteries are designed to be used until their energy is spent, and then disposed of. The chemical reaction is not reversible, so they are not rechargeable. Examples of primary batteries include alkaline, lithium (metal), silver-oxide, and zinc-carbon batteries.
- Secondary batteries are designed to be recharged and used multiple times. Their chemical reaction can be reversed by applying electric current to the cell (e.g. charging your cell phone). The original chemical reactants are regenerated, and they can be used again. Examples include lithium-ion and lithium polymer, lead acid, nickel-cadmium, and nickel-metal hydride batteries. During charging, the roles of the anode and cathode (direction of current flow) are reversed.

Nickel Metal Hydride rechargeable batteries
Lithium-Ion VS Lithium Metal Batteries
It is important to note the difference between lithium-ion and lithium metal batteries. Despite similar names, the chemistry in the batteries varies greatly, and they have unique hazards. Lithium metal batteries are primary, and lithium-ion batteries are secondary.
In lithium-ion batteries, the cathode contains the lithium based compounds (lithium cobalt oxide, lithium iron phosphate, lithium nickel manganese cobalt oxide, etc.) while the anode is graphite. The electrolyte is an organic medium like lithium perchlorate in ethylene carbonate. No elemental lithium is present.
By contrast, in lithium batteries, lithium metal is the anode, and another chemical is the cathode (e.g. manganese dioxide). The electrolyte is an organic medium like lithium perchlorate in propylene carbonate.

Lithium-ion battery pack

Lithium metal batteries
It is important to know which sort of battery you are working with. Due to the presence of elemental lithium, the fire hazard differs from lithium-ion batteries.
Lithium-Ion And Lithium Polymer Batteries
In recent years, lithium-ion and lithium polymer (Li-Po) batteries have become widespread in their use and applications. Multiple incidents involving lithium-ion batteries have been documented and covered by the media (e.g. cell phone battery fires). These are often the result of manufacturing defects. Lithium-ion battery failures have resulted in fires on campus and at other universities. Many of these have been caused by misuse.
Lithium-ion and lithium polymer batteries are widely used in consumer electronics, and are used across campus. Cellphones, power tools, laptops, and even drones depend on lithium-ion or lithium polymer batteries. Due to the similarities between lithium-ion and lithium polymer batteries, similar handling should be considered during charging and use.
Lithium-ion battery packs found in consumer electronics (e.g. laptops) contain an internal Battery Management System that controls the charging process. Be aware of situations that can lead to the battery becoming damaged, such as physically damaging the device or storing it in a hot environment. Using the charger provided with the device and following the manufacturer’s requirements will require no additional input from the user.

Lithium-ion laptop battery

LiPo battery pack. The manufacturer clearly states requirements on the label.
LITHIUM-ION BEST PRACTICES
When handled correctly, the risks of an incident involving a lithium-ion or LiPo battery are low. Follow your groups Laboratory Safety Plan and Standard Operating Procedures (SOPs) when working with batteries. Follow these recommendations when developing your groups SOPs:
- Purchase batteries from reputable manufacturers and suppliers. Batteries should be certified to meet the safety requirements of a consensus based safety standard. Examples of these standards include Underwriter’s Laboratory (UL) 1642 Standard for Lithium Batteries and the International Electrotechnical Commission (IEC) 61960.
- Always follow the manufacturer’s information supplied with the battery. This includes recommended storage requirements, such as the temperature and voltage (e.g. an 11.1 V three cell battery pack stored at 40% charge measured with a meter), as well as charging requirements.
- Only use a charger that is rated for your lithium-ion or LiPo battery. The nominal cell voltage of a typical cobalt based lithium-ion battery is 3.60V/cell, with a max charge voltage of 4.2V and discharge at 3.0V.
- Avoid overcharging or over-discharging the battery, this can stress the battery and lead to a failure. Overcharging can cause the cathode to become unstable, producing carbon dioxide, eventually leading to a failure and possible fire.
- Use a fire retardant battery bag (e.g. LiPo-Guard) when charging or storing batteries. Never leave a charging battery unattended, and take action if anything out of the ordinary is observed (e.g. bulging). When using a battery pack, make sure your charger is capable of monitoring the condition of individual cells so no one cell becomes overcharged.

LiPo battery and charger

Fire retardant charging bag and LiPo battery
If you notice any bulging, punctures, excess heat, smells, or anything out of the ordinary, disconnect the battery from the charger if it is safe to do so, and see procedures for handling bulging and swelling batteries below.
Be aware that your battery may differ from the parameters stated above. You should always follow the parameters for your battery.
When storing batteries for more than a few days, store the battery at 40% charge, or follow the manufacturer’s instructions. Many chargers have a storage mode that will either charge or discharge a battery to the recommended storage voltage.
It should be kept in a cool, dry place. Storing a battery at too high or low of a temperature can cause irreversible damage. Ideally batteries should be stored near 15°C (~59°F), this will help prolong the life of the battery. Batteries can typically be used at extreme temperatures (-20 to 60°C), but storing batteries in these conditions should be avoided. Use a fire retardant battery bag for storage when possible, and do not store them near flammable materials or heat sources. Protect the terminals during storage with an electrically insulating material (e.g. rubber caps). Storing multiple batteries together could cause a short circuit if the terminals come into contact.
LITHIUM-ION AND LIPO BATTERY FAILURE
Lithium-ion battery cells contain a metal oxide at the cathode (e.g. lithium cobalt oxide), the anode is graphite, and the electrolyte is a solvent containing a lithium salt (e.g. lithium perchlorate or lithium hexafluorophosphate in ethylene carbonate). The solvent in the electrolyte is flammable, and is the source of lithium-ion battery issues. Little to no elemental lithium is present in lithium-ion batteries. Lithium-ion battery packs (such as those used in laptops) include multiple cells. The Department of Energy illustrates how a lithium-ion battery works here.
When handled correctly, the risk of a fire is low. Misuse of lithium-ion batteries are what typically lead to a failure. This can include thermal abuse, mechanical damage, and electrical abuse.
Thermal Abuse - This is the result of storing or using a battery outside of its recommended temperature range.
Mechanical Abuse – Mechanical abuse is the result of physically damaging a lithium-ion battery. This includes dropping, crushing, vibrations, and water damage.
Electrical Abuse – Electrical abuse involves mismanagement of the recommended electrical conditions. This includes overcharging or over-discharge and using the incorrect charger for the battery in question.
Many of these scenarios can lead to the internal plastic separator between the anode and cathode becoming damaged, allowing the anode and cathode to come into direct contact. This leads to elevated temperature in the battery cells and the generation of gases from the electrolyte.
As gas (oxygen) and heat is generated by the battery, you may notice the cell starting to bulge or swell. If left unchecked, this can lead to thermal runaway in the cell, continuing to heat and generate more gas. Eventually the gas can lead to a rupture in the cell. With the elevated temperature, flammable electrolyte, and oxygen, a fire can occur.
Further complicating the fire is the potential for a chain reaction when multiple cells are present. A fire that occurs from a failure in one cell can damage a neighboring cell, which can ignite independent of the first cell. This can occur during the original fire, shortly after the first cell burns out, or even hours later.
LITHIUM-ION AND LIPO BATTERY SWELLING


The LiPo battery packs in these pictures are identical. The pack on the left is visibly bulging.
If you notice bulging/swelling, punctures, or the battery has suffered damage due to any abuse (even with no visible damage), follow these steps:
- Don appropriate PPE (eye protection, gloves, lab coat)
- Place the battery in a bucket (metal or hard plastic)
- Place the bucket away from any flammable materials or heat sources
- Fill the bucket with a salt water solution to discharge the battery
- You will notice bubbles starting to form in the salt water solution
- Allow the battery to sit in the salt water solution to fully discharge (at least 3 days)
- Remove the battery from the solution, dry it, and check the voltage using a multimeter. It should read near 0 V.
- If the voltage is still too high, repeat the above steps and check it again
- After the battery has been fully discharged, protect the terminals with tape, and request the battery for disposal through DRS. Use UI# 7580 for lithium-ion and UI# 205346 for LiPo.
Indicate that this was a damaged battery that was fully discharged prior to disposal in the waste pickup notes.
LITHIUM-ION AND LIPO BATTERY FIRES

Aftermath of a LiPo battery fire. The remains of the battery are at the center of the image.
If a battery is smoking, or flames are present, this is an emergency. Always call 911 during an emergency situation, and evacuate the immediate area. Contact DRS when it is safe to do so.
Be aware of the potential gases that may be present during a lithium-ion battery fire. Carbon monoxide and dioxide can be released, and gases caused by the decomposition of the lithium hexafluorophosphate can be generated as well. This can include hydrogen fluoride (HF) gas.
DRS offers Fire Extinguisher training here. Personnel that are trained in the use of fire extinguishers may attempt to extinguish a fire if it is safe to do so. Remember that lithium-ion and LiPo batteries do not contain lithium metal, and the solvent in the electrolyte is what burns during a lithium-ion battery fire. A standard ABC Dry Chemical or Carbon Dioxide fire extinguisher is suitable for handling small lithium-ion battery fires. Smothering the battery with sodium bicarbonate can also extinguish a small lithium-ion battery fire.
Battery Disposal
Request unwanted batteries for disposal through the DRS Waste Management App. DRS will recycle batteries whenever possible. Lithium metal, lithium-ion and lithium polymer batteries must have their terminals (or connections) protected during shipment to prevent short circuits; non-conductive tape can be used for this purpose.
Batteries should be separated and requested for disposal by type. You may add like batteries to a zip lock bag or box and request them as 1 item with weight being in total pounds (not to exceed 35 pounds). Use one of the following UI#’s when requesting your batteries for disposal:
- UI# 7575 - Alkaline Batteries
- UI# 7602 - Lead-Acid Batteries
- UI# 7580 - Lithium Metal Batteries
- UI# 9111 - Lithium-Ion Batteries
- UI# 205346 - Lithium Polymer Batteries
- UI# 5230 - Mercury Batteries
- UI# 9109 - Nickel Metal Hydride Batteries
- UI# 150207 - Zinc-Carbon Batteries
If your batteries are not listed above, please select UI# 1 and specify the type of battery in the Waste Description field.
Resources
http://batteryuniversity.com/
https://www.nfpa.org/News-and-Research/Fire-statistics-and-reports/Research-reports/Hazardous-materials/Lithium-ion-batteries-hazard-and-use-assessment
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5577247/
