DCM registration is now open (Click link to access the registration page)
Introduction
Methylene chloride, also known as dichloromethane (DCM), is a common solvent used in laboratory operations and in products such as degreasers and paint strippers. The volatility of this solvent makes inhalation a primary route of exposure to cause health effects.
Many common chemical products that contain >0.1% DCM are currently used on campus. In addition to reagent grade DCM, DCM is included in a variety of products including adhesives and sealants, metal degreasers, automotive care products, lubricants, hobby glue, strippers, brush cleaners, etc. DCM and DCM-containing products are commonly used in the laboratory for extractions, chromatography, tissue clearing, and many other processes.
DCM health effects
Health risks associated with DCM include acute and chronic exposure via dermal contact and inhalation. DCM is considered a carcinogen (cancer-causing substance), an acute neurotoxin, and chronic exposure can affect liver function. Target organs include the eyes, skin, cardiovascular system, and central nervous system. Acute symptoms include irritation to the eyes and skin, weakness and exhaustion, drowsiness, dizziness, numbness, and nausea. EPA's full risk evaluation detailing the health effects can be accessed here. OSHA also provides resources regarding DCM.
Regulatory Oversight
In June 2016, Congress amended the Toxic Substances Control Act (TSCA) to assess and address risks from chemicals in commerce. Per TSCA (Section 6), the EPA is required to address by rule any unreasonable risk of injury to health or the environment as part of a TSCA risk evaluation and apply requirements so the chemical no longer presents an unreasonable risk. Methylene chloride was one of the first chemicals identified for risk evaluation. The final risk evaluation was published in 2022 and the final rule was published in April 2024. The EPA determined that methylene chloride presented an unreasonable risk of injury to the health of workers, consumers, and bystanders in 52 of 53 conditions of use. The rule applies to all products that contain >0.1% DCM.
The health hazards associated with DCM are well established and it is currently regulated by the Occupational Health and Safety Administration (OSHA) in the workplace. This was most recently updated in 1997. The updated exposure limits for DCM are listed below and are much lower than the OSHA exposure limits. More information about exposure limits and exposure assessments can be found here.
| OSHA (1997) | EPA (2024) | |
| 8-hour TWA | 25 ppm PEL | 2 ppm ECEL |
| 15-minute STEL | 125 ppm | 16 ppm |
| Action Level | 12.5 ppm | 1 ppm |
These new exposure limits present more conservative (e.g. more protective) values for workers. Additionally, the new exposure limits extend to all members of the University community, including employees, students, visitors, and members of the public.
Campus Action
Where feasible, eliminating DCM use in the laboratory or substituting for a less hazardous material are the preferred approaches to protect from exposure. If DCM is not used or is substituted with a less hazardous material, the requirements of the standard are met. Eliminating or substituting DCM removes the unreasonable risk of exposure noted in the standard and further administrative burden. If DCM use cannot be eliminated or substituted, laboratories must document why this is not possible. DRS will work with researchers to find alternatives when feasible.
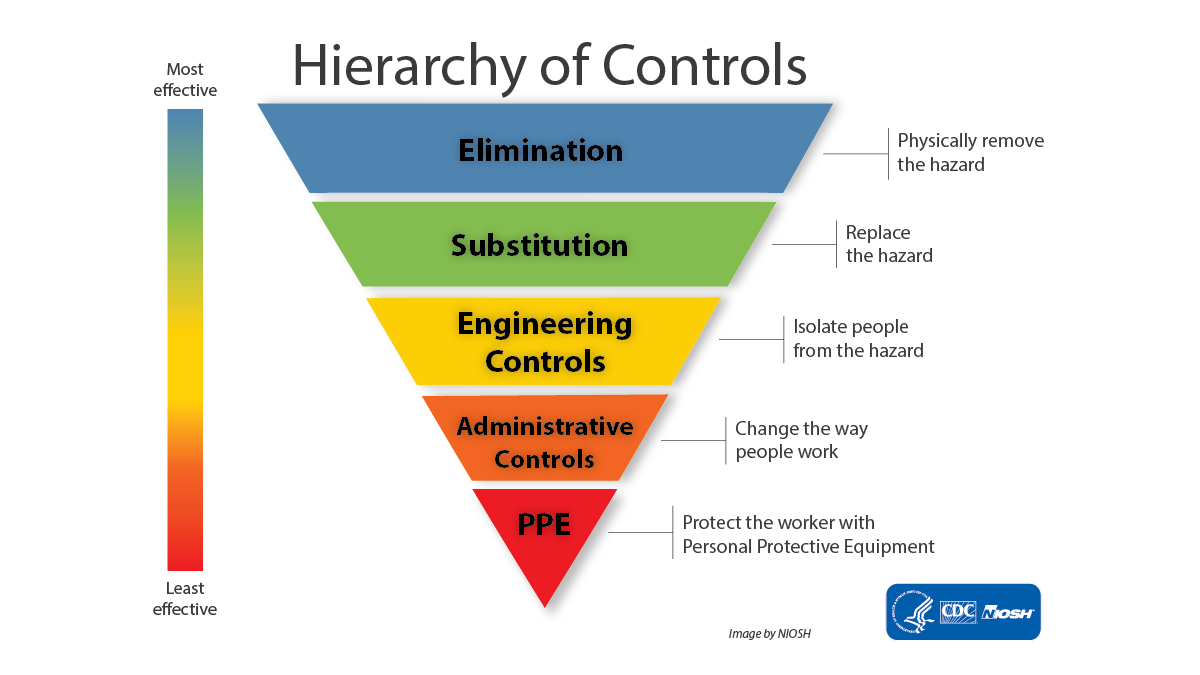
Elimination or substitution are not always feasible, and some uses of DCM must continue to further our research and education missions. This will require the development of a DCM Exposure Control Plan, including personal breathing zone exposure monitoring. DRS is preparing a template for the Exposure Control Plan that will utilize our existing registration database. Users must select control options according to the Hierarchy of Controls.
The registration process will function as the Exposure Control Plan and ensure all necessary elements are met. DRS will then perform personal breathing zone exposure monitoring utilizing established Industrial Hygiene methods, equipment, and accredited labs. Results will be compared to the new exposure limits published in the standard, which will dictate any further actions and the future sampling schedule. These results will be documented in the registration database. DRS will prepare program information as part of the Workplace Chemical Protection Program detailing the campus approach to the new standard.
Elements of the Workplace Chemical Protection Plan
- Exposure Limits;
- Initial and periodic exposure monitoring;
- Establishment of a regulated area;
- Development and communication of an exposure control plan;
- Respirator selection criteria and personal protective equipment (PPE);
- Training;
- Recordkeeping;
- Downstream notification.
DRS will maintain the campus Workplace Chemical Protection Plan.
Elements of the Exposure Control Plan
- Identification of possible exposure control measures and the rationale for using or not using available exposure controls in the sequence described by hierarchy of controls;
- For the exposure controls not selected, documentation of the efforts identifying why these are not feasible, not effective, or otherwise not implemented;
- A description of actions the owner or operator must take to implement exposure controls selected, including proper installation, regular inspections, maintenance, training, or other steps taken;
- A description of regulated areas, how they are marked, and persons authorized to enter the regulated areas;
- A description of activities conducted by the owner or operator to review and update the exposure control plan to ensure effectiveness of the exposure controls, identify any necessary updates to the exposure controls, and confirm that all persons are properly implementing the exposure controls; and
- An explanation of the procedures for responding to any change that may reasonably be expected to introduce additional sources of exposure to methylene chloride, or otherwise result in increased exposure to methylene chloride, including procedures for implementing corrective actions to mitigate exposure to methylene chloride.
The DRS registration process will manage the Exposure Control Plan for your laboratory.
Registration
The following questions and items will be listed in the registration process. Facility and user information will utilize the existing DRS database. In addition to the locations and personnel working with DCM, the following question will be asked:
- Controls-Elimination:
- Why is methylene chloride necessary for this process? Can the process be completed without it? Explain
- Controls-Substitution:
- Is there a suitable substitution for methylene chloride? Explain why or why not.
- Description of Use:
- Describe how methylene chloride is used. Include details on the control measures adopted to limit employee exposure.
- Approximately how long does the process take?
- How often (frequency) is the process performed?
- List lab equipment used in this procedure.
- Controls-Engineering:
- List all the safety equipment you will be using for this procedure.
- If a chemical fume hood was chosen, is it part of the annual inspection program?
- Controls-Administrative:
- What parts of the process/task pose the highest risk of exposure to individuals (inhalation/dermal exposure)? DRS risk assessment page link.
- What work practices are employed to minimize the risk of exposure during the task?
- Controls-Personal Protective Equipment (PPE):
- Are you anticipating splash/incidental contact or extended contact with DCM during your task?
- Choose Personal Protective Equipment (PPE) used for the procedure.
- Additional Requirements:
- When changes occur in procedures or workplace practices for methylene chloride use in a manner that would impact exposure levels, we will reach out to DRS for additional evaluation and monitoring.
- We will review and update our registration as necessary and least every 5 years.
- All users of DCM will be trained on Standard Operating Procedures involving the use of DCM prior to initial job assignments.
Include any additional details not captured in the above questions that will help determine risk of exposure to personnel.
Deadlines
- May 5, 2025 - Initial Monitoring
- August 1, 2025 - Exposure Limits and Dermal Protections
- October 30, 2025 - Exposure Control Plan
DRS will be communicating with users regularly and updating this website. Compliance with this standard is a work in progress. Stay tuned for more information, including information about the registration process.