White House Issues Executive Order
On May 5, 2025, an Executive Order on Improving the Safety of Biological Research was issued which pauses the 2024 DURC-PEPP policy that was to be implemented on May 6, 2025. The Director of the Office of Science and Technology Policy (OSTP) and the National Security Advisor will work with funding agencies to develop a new policy within 120 days to replace the 2024 DURC-PEPP policy. In the interim, previous USG DURC policies and HHH P3CO Framework remain in place. Updates will be made as they are announced.
Oversight of DURC-PEPP Policy
The US Government is committed to improving the safety of biological research to assure that experiments do not intentionally or unintentionally enhance a pathogen or toxin such that it may pose a significant threat to public health, agriculture, food security, economic security, or national security.
Dual Use Research of Concern (DURC) is life sciences research that, based on current understanding, can be reasonably anticipated to provide knowledge, information, products, or technologies that could be misapplied to do harm with no, or only minor, modification to pose a significant threat with potential consequences to public health and safety, agricultural crops and other plants, animals, the environment, or national security.
Pathogen with Enhanced Pandemic Potential (PEPP) is a type of pathogen with pandemic potential (PPP) resulting from experiments that enhance a pathogen’s transmissibility or virulence, or disrupt the effectiveness of pre-existing immunity, regardless of its progenitor agent, such that it may pose a significant threat to public health, the capacity of health systems to function, or national security. Wild-type pathogens that are circulating in or have been recovered from nature are not PEPPs but may be considered PPPs because of their pandemic potential.
The United States Government released a new policy and implementation guidance for research involving DURC-PEPP. This policy supersedes previous 2012 and 2014 USG DURC policies and the 2017 HHS Framework for Guiding Funding Decisions about Proposed Research Involving Enhanced Potential Pandemic Pathogens (HHS P3CO Framework) beginning May 6, 2025.
Scope of Oversight
Federally funded intramural or extramural research that meets the scope of Category 1 or Category 2 research within this policy is subject to federal and institutional oversight.
Category 1 Research
Category 1 Research meets all three criteria listed:
- It involves one or more of the biological agents and toxins specified in Appendix C of the implementation guidance.
- Exceptions made: HIV, HTLV, SIV, Mtb (including mycobacterium bovis), Clade II of MPVX viruses unless containing nucleic acids coding for clade I MPVX virus virulence factors, vesicular stomatitis virus, Coccidioides immitis, C. posadasii, Histoplasma capsulatum, and H. capsulatum var. Duboisii.
- In the event no Risk Group or Biosafety Level has been assigned to an agent, for example in the case of a newly emerging pathogen or chimeric agent, the PI and IBC should perform a risk assessment to determine the appropriate Biosafety Level for handling the agent. Agents that would require BL-3 or BL-4 are subject to this policy. The University of Illinois Urbana-Champaign does not have facilities to host BL-3 and BL-4 research.
2. It is reasonably anticipated to result, or does result, in one of the experimental outcomes specified:
a. Increase transmissibility of a pathogen within or between host species;
b. Increase the virulence of a pathogen or convey virulence to a non-pathogen;
c. Increase the toxicity of a known toxin or produce a novel toxin;
d. Increase the stability of a pathogen or toxin in the environment, or increase the ability to disseminate a pathogen or toxin;
e. Alter the host range or tropism of a pathogen or toxin;
f. Decrease the ability for a human or veterinary pathogen or toxin to be detected using standard diagnostic or analytical methods;
g. Increase resistance of a pathogen or toxin to clinical and/or veterinary prophylactic or therapeutic intervention;
h. Alter a human or veterinary pathogen or toxin to disrupt the effectiveness of preexisting immunity, via immunization or natural infection, against the pathogen or toxin; or
i. Enhance the susceptibility of a host population to a pathogen or toxin.
3. Based on current understanding, the research institution and/or federal funding agency assesses that the research constitutes DURC as defined above.
Category 2 Research
Category 2 Research meets all three criteria listed:
1. It involves, or is reasonably anticipated to result in, a pathogen with pandemic potential (PPP: a pathogen that is likely capable of wide and uncontrollable spread in a human population and would likely cause moderate to severe disease and/or mortality in humans.)
2. It is reasonably anticipated to result in, or does result in, one or more of the experimental outcomes or actions specified:
a. Enhance transmissibility of the pathogen in humans;
b. Enhance the virulence of the pathogen in humans;
c. Enhance the immune evasion of the pathogen in humans such as by modifying the pathogen to disrupt the effectiveness of pre-existing immunity via immunization or natural infection; or
d. Generate, use, reconstitute, or transfer an eradicated or extinct PPP, or a previously identified PEPP.
3. Based on current understanding, the research institution and/or federal funding agency assesses that the research is reasonably anticipated to result in the development, use, or transfer of a PEPP or an eradicated or extinct PPP that may pose a significant threat to public health, the capacity of health systems to function, or national security.
Assessment of Research for DURC-PEPP
The Responsible Individual (e.g. Principal Investigator) is required to make an assessment at the proposal state and continuously throughout the entire course of the research to identify whether research is reasonably anticipated to be within the scope of Category 1 or Category 2 research. The DURC-PEPP policy applies to research funded or sponsored by grants, contracts, cooperative agreements, and other agreements and transactions issued on or after May 6, 2025.
Investigators must notify the campus Institutional Contact for DURC-PEPP (ICDUR: Linda Arseneau; lmarsene@illinois.edu) and the Division of Research Safety (DRS@illinois.edu) to refer the research to the Institutional Review Entity (IRE) if their assessment of proposed or continued research identifies the potential of Category 1 or Category 2 research. If identification occurs during the course of experiments, work must halt until the IRE review has been completed. Investigators must also notify the funding agency and be prepared to develop a risk mitigation plan.
The Institutional Biosafety Committee (IBC) requires registration of all pathogens (human, animal, or plant), biotoxins, and use of recombinant or synthetic nucleic acid molecules. The IBC has implemented questions to screen if research may constitute a potential DURC-PEPP project and determine if further review by the IRE is required.
Federal funding agencies have the discretion to request additional information or review of individual research proposals or projects to determine whether they may fall within scope of Category 1 or Category 2 research.
If an investigator identifies research that may be within the scope of Category 1 or Category 2, or a funding agency requests review, the campus IRE reviews the PI’s assessment and confirms whether proposed or ongoing research is within the scope of Category 1 and/or Category 2 research.
Institutional Review Process of Potential DURC-PEPP Projects
To comply with the U.S. government policy, a campus Institutional Review Entity (IRE) is established to conduct assessments of projects which may constitute DURC-PEPP. It is the responsibility of the IRE to assess research referred by the PI to determine whether it meets the threshold to be designated as Category 1 or Category 2 research. The federal funding agency is responsible for evaluating and verifying the IRE’s assessment.
IRE reviews are initiated in one of two ways:
- Requested by the PI or granting agency for proposed grants.
- Through IBC screening questions that check materials and experimental outcomes.
The formal review starts with investigator submission of the IRE Assessment Form to the campus ICDUR or DRS@illinois.edu. The IRE will assess whether the research meets the criteria of Category 1 or Category 2 research. For research determined to be Category 1 or Category 2, the IRE will work with the PI to draft a risk-benefit assessment and risk mitigation plan for the conduct and communication of the research to be submitted to the applicable U.S. government funding agency.
PIs are required to obtain IBC approval prior to initiation of experiments with material requiring registration. If the IRE determines work meets the criteria for Category 1 or Category 2 research, the approved risk-mitigation plan must be uploaded to the applicable IBC project(s).
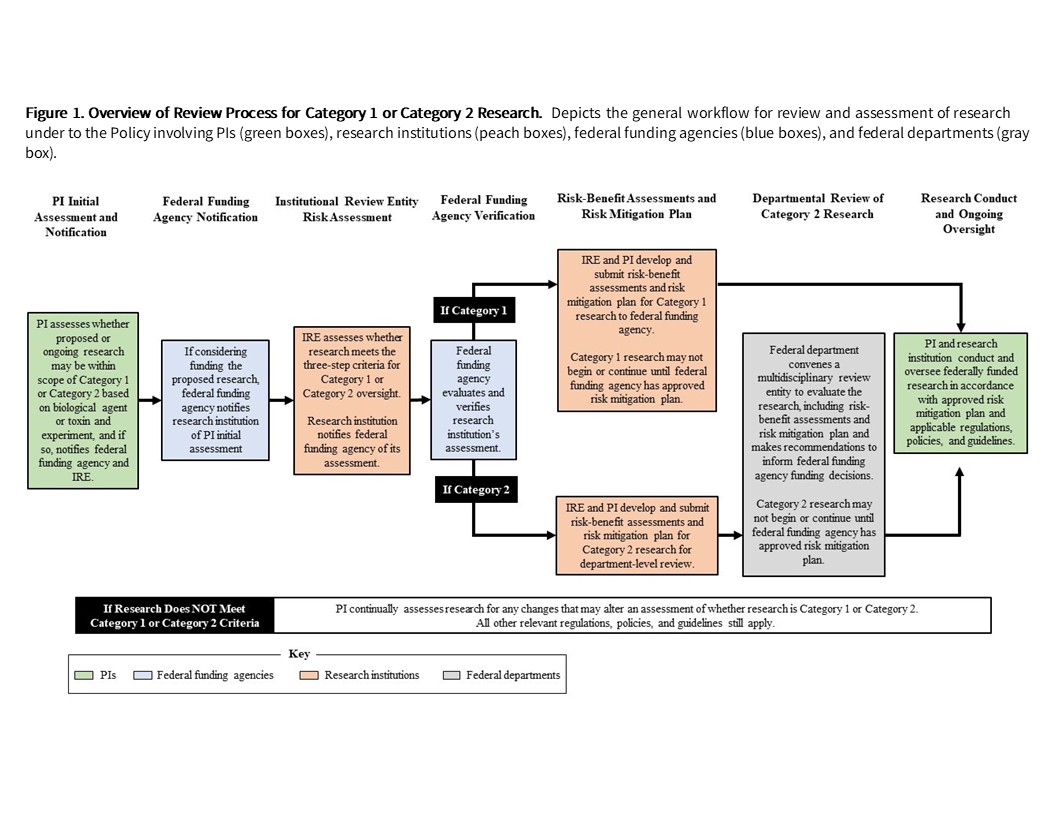
An overview of the Review Process can be found in Figure 1 below:
Training
The CITI Dual Use of Research of Concern training is required by:
- All PIs, staff, and students whose research is subject to the policy
- IRE members
- Administrative staff supporting the IRE.
Institutional Contact for DURC-PEPP (ICDUR)
Linda Arseneau
Associate Director, Biological Safety Officer
lmarsene@illinois.edu
217-244-1939
Definitions
A list of definitions are found in Appendix A of the Implementation Guidance
References
NIH Implementation Information
U.S. Department of Health & Human Services: DURC-PEPP Oversight Framework
U.S. Department of Health & Human Services: High Consequence Research Oversight