BSC Protections
Whenever aerosol generating procedures are used, such as: pipetting, centrifuging, grinding, blending, shaking, mixing, sonicating, opening containers of infectious materials, inoculating animals intranasally, and harvesting infected tissues from animals, the use of a Biological safety cabinet (BSC) as an engineering control greatly reduces exposure to aerosols.
Class II BSCs are designed for:
- Personnel Protection: Protects personnel from harmful agents inside the BSC.
- Product Protection: Protects the work, product, experiment, or procedure performed in the BSC from contaminants in the laboratory environment or from cross contamination inside the cabinet.
- Environmental Protection: Protects the environment from contaminants contained in the BSC.
Anatomy and Function of a BSC
This cross-section diagram of the most common type of BSC found on campus may also be found in the Biosafety in Microbiological and Biomedical Laboratories (BMBL) 5th Edition and will help familiarize users with the basic structure of a BSC.
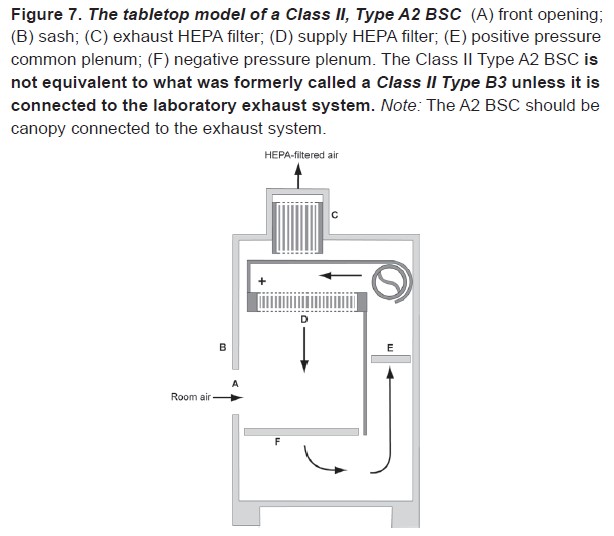
An internal fan draws room air into the front grille of the cabinet, which then flows through a High Efficiency Particulate Air (HEPA) filter to provide downward, particulate-free air to the work surface. This airflow provides product protection by minimizing the chances of cross-contamination across the work surface of the cabinet. The downward moving air “splits” as it approaches the work surface as a fan draws part of the air to the front grille, providing personal protection from contaminated work surface air, and the remainder to the rear grille. The air is then either recirculated through the HEPA filter back into the work zone or exhausted. Because cabinet exhaust air is also passed through a HEPA filter, it is particulate-free (environmental protection), and may be recirculated to the laboratory. HEPA filters are composed of one continuous pleated sheet of borosilicate fibers woven into a crosshatched design. A HEPA filter can trap up to 99.97% of particles with dimensions equal to or greater than 0.3 micron. A comprehensive description of BSC types, performance characteristics, and applications can be found in the BMBL Appendix A: Primary Containment for Biohazards: Selection, Installation and Use of Biological Safety Cabinets (1).
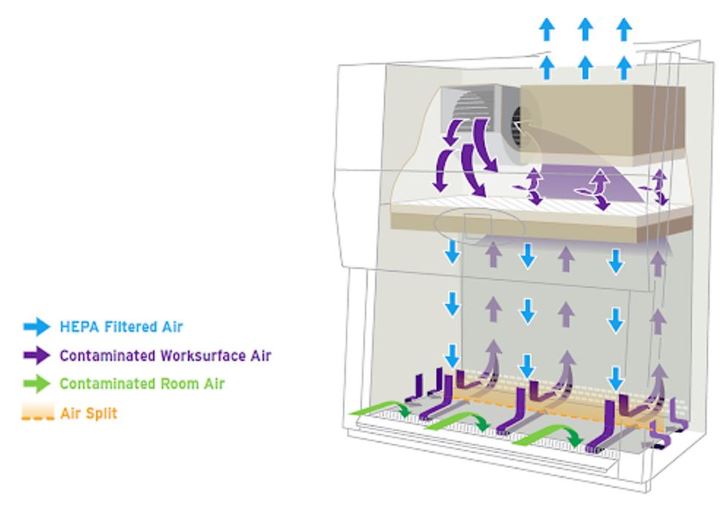
HEPA filters are present in all classes of BSCs. A HEPA filter removes only particulates (including microorganisms), not vapors or gases, from the air. HEPA filters are the main line of defense against contamination in BSC construction (1, 8, 9).
Most BSCs on Campus are Class II Type A2 and are not for Chemical Use:
It is important to remember that HEPA filters are effective at trapping particulates and thus infectious agents but do not capture volatile chemicals or gases. Volatile or toxic chemicals should not be used in Class II Type A cabinets without ducting because vapor build-up inside the cabinet presents a fire or explosion hazard. In addition, this type of cabinet recirculates air from the cabinet workspace into the room, potentially exposing the operator and other room occupants to toxic chemical vapors.
Only Type A2-exhausted or Types B1 and B2 BSCs exhausting to the outside should be used when working with volatile, toxic chemicals, but amounts must be limited.
Before selecting a cabinet, potential users must evaluate their program and match specific requirements with the appropriate equipment. The Division of Research Safety (DRS) can assist researchers with these evaluations.
BSC Placement
An air current of 1 mile per hour is enough to disrupt the airflow inside of a BSC so it is very important that undisturbed spaces are maintained around the BSC. The following parameters summarized below are recommended by the NIH (5, 6):
- Maintain an undisturbed space of 40” from the face of the BSC.
- Maintain a distance of 12” to walls and 40” to bench tops perpendicular to the BSC.
- Maintain a distance of 120” between BSC’s on opposing walls and allow for 40” between BSCs on the same wall.
- Place BSCs at least 80” from opposing walls.
- Placing BSCs near doorways is never recommended.
Certification Requirement
To ensure that BSCs are providing adequate personnel and environmental protection, on-site testing and certification of BSCs is required:
- At the time of installation;
- At least annually thereafter;
- At any time the BSC is moved.
Principal Investigators (PIs) are responsible for ensuring that BSCs in their facilities are certified on an annual basis. Most departments have a designated safety contact who will coordinate the certification of BSCs with a qualified vendor. If there is no designated safety contact for the unit, the PI is responsible for contacting a vendor and scheduling the certification. Once certification is completed, cabinet information should be updated in the DRS safety cabinet management system.
PIs, Safety Contacts, and Cabinet Managers will receive email reminders 30 days prior to the expiration of a BSC and weekly thereafter until a certification is entered or the cabinet status is changed to in-storage or not in use. Email reminders will continue until cabinet information has been updated in the safety cabinet management system.
Accredited Certifiers
Labs must use accredited certifiers with a Certificate of Liability on file. DRS maintains a list of qualified vendors that have certified or repaired BSCs on campus, which is available upon request.
Other accredited certifiers can be located by using the following link: NSF
Note: P-Cards and i-Cards cannot be used as payment for services or repairs of biosafety equipment. Biosafety Cabinet certifications or repairs require a purchase order (P.O.), either “Regular” or “Standing,” in Banner for payment. If you have questions about obtaining a P.O., contact your business office.
Use of Uncertified BSCs
If a BSC is no longer certified (due to either certification expiration or failure) it must not be used as a primary safety device.
Why?
An uncertified biosafety cabinet with the blowers running may have defective filtration and disseminate potentially harmful material throughout the environment. Any work with pathogens or potentially infectious materials, even those classified as Risk Group 1, should not be performed in an uncertified cabinet. Thus, using an uncertified BSC is strongly discouraged in laboratories, particularly where pathogens or potentially infectious materials are also present because the potential for, and risks associated with, using the equipment incorrectly are increased.
Occasionally, there are requests to work with non-pathogenic materials (e.g., plant or certain animal tissue cultures) in an uncertified cabinet. For those who wish to work in an uncertified BSC, contact DRS for assistance with developing a risk assessment to determine if the proposed use would qualify for an exemption from the certification requirement. This should be considered only if the situation can be carefully managed. The cabinet should have a warning sign, and occupants of the laboratory should be trained on the limitations and potential hazards of using the equipment incorrectly.
Open Flames and Gas Connections in the BSC
Gas connections to a BSC are generally not permitted.
Why?
Open flames in BSCs:
- Create turbulence in the airflow (for more information, read here) thus disrupting the pattern of air supplied to the work surface. Not only does this compromise protection to both the worker and the experiment, but heat in the BSC increases the likelihood of cross contamination.
- Present a potential fire or explosion hazard. All flames must be turned off before disinfectants are used; particularly where 70% ethanol/isopropanol is being used as a disinfectant.
- Cause excessive heat build-up, which may damage HEPA filters and compromise the cabinet’s integrity.
- May inactivate the manufacturer’s warranty.

If your research requires working with a flame in a BSC:
- A written justification for specialized, limited-duration work must be added to an IBC.
- Routine “flaming” is not considered adequate justification.
- Approval must be given prior to using flames in a BSC.
Note: The update to the IBC should include a description of this use and why a non-flame alternative cannot be used. If IBC approval is given, approved gas connections should have an additional shut-off valve installed outside of the BSC.
Acceptable devices for flaming in a BSC include touch plate micro burners, which provide a flame on demand, or electric furnaces. These heat sources should be placed in the back third of the BSC.
Ultraviolet Lamp Usage
The Center for Disease Control (CDC) and the National Institute of Health (NIH) agree that UV lamps are not recommended or required in BSCs. Proper use and cleaning of BSCs negates any need for the use of UV lamps. If using UV light as a secondary decontamination method follow the following guidelines:
- UV lamps must be turned off when the room is occupied to protect eyes and skin from UV exposure, which can burn the cornea and cause skin cancer. If the BSC has a sliding sash, close the sash when operating the UV lamp.
- Numerous factors affect the activity of the germicidal effect of UV light.
- If installed, UV lamps must be cleaned weekly to remove any dust and dirt that may reduce their germicidal effectiveness.
- Lamps should be checked weekly with a UV meter to ensure that the appropriate intensity of UV light is being emitted: Five minutes after the lamp has been turned on, the sensor of the UV meter is placed in the center of the work surface. The radiation output should not be less than 40 microwatts per square centimeter at a wavelength of 254 nanometers (nm).
Use of Biological Safety Cabinets
The safety guidance below describes the proper use of BSCs in the laboratory. Proper use of BSCs protect the user and other personnel in the laboratory space from potential exposure to biohazardous materials. The PI is responsible for developing and implementing standard operating procedures (SOPs) for safely conducting research in a BSC.
Preparing BSC for Work
- Confirm that the BSC is currently certified (within the last 12 months) for use and is operating properly prior to beginning work.
- If in use, turn off germicidal UV light and check weekly UV output log.
- Ensure sash is set in the correct operating position.
- Cabinet blowers will need to operate for 5-15 minutes before beginning work to allow the BSC to purge particulates. Check manufacturer instructions for appropriate purge time.
- Only prepare materials required for a particular activity & remove unnecessary materials from the BSC before beginning work.
- Adjusted chair/stool height so that armpits are level with the bottom of the view screen or sash.
Personal Protective Equipment
- Lab coats must be buttoned. If back closing laboratory gowns are utilized for greater protection, they must be tied. Cuffed lab coats are recommended to facilitate easier donning. Gloves should be pulled over the wrists of lab coats, not worn inside the sleeve.
- Additional PPE may be required, reference your laboratory’s IBC registration to determine if additional requirements may be necessary.
Working in the BSC
- Only materials and equipment needed for the immediate work should be placed in the BSC.
- Move arms in and out of the cabinet slowly, perpendicular to the face opening, to limit disruption of the protective air flow patterns.
- Manipulation of materials inside the cabinet should be delayed for 1 minute after placing hands/arms inside the cabinet to allow the air to stabilize and to “air sweep” arms.
- Perform all operations on the work surface and at least 4 inches from the front grille.
- Do not rest arms on front grille unless the BSC is specifically equipped with features that permit this action. Doing so allows room air to flow directly into the work area rather than being drawn through the front grille. Instead, work with both arms raised slightly.
Placement of Materials in the BSC
- To minimize frequent in/out arm movement, consider using: horizontal discard trays containing an appropriate chemical disinfectant; aspiration devices; and collection vessels for tips/serological pipettes.
- All materials should be placed as far back in the cabinet as practical, toward the rear edge of the work surface and away from the front grille of the cabinet.
- Materials and supplies should be placed in the cabinet in such a way as to limit the movement of dirty items over clean ones. Make sure that active work flows from the clean to contaminated area across the work surface.
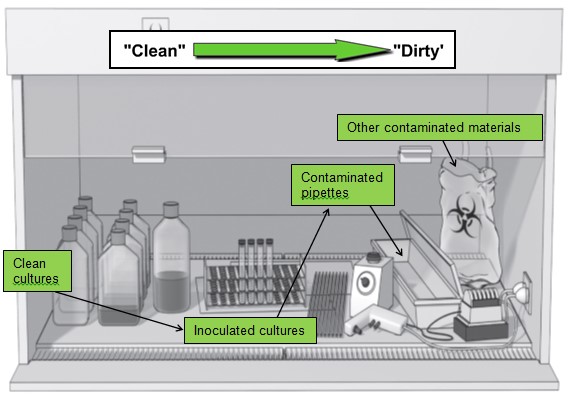
- Do not use equipment or store supplies inside the BSC that may disrupt the protective BSC airflow pattern. If large equipment must be placed inside the BSC, place it as far back in the BSC as practical. Equipment should be placed behind the air split.

- Do not block the front grille with papers or other materials. If absorbent materials are used to aid in cleanup and spill containment, make sure it is not placed on the front grille.

Liquid Waste
If using a vacuum, a vacuum flask system is required to provide protection to the central building vacuum system and to personnel who service the equipment.
Connect the primary flask to an overflow collection flask and then to an in-line HEPA filter. Both flasks shall contain an appropriate disinfectant (e.g. bleach) for the material used. Traps should be routinely emptied after waste has had proper contact time with the correct concentration of disinfectant (e.g. 10% final concentration of bleach prior to disposal for at least 1 hour contact time due to high organic load).

The vacuum flasks may be set up on the floor beneath or next to the BSC, using a secondary container to contain the flasks. If traps are at eye level in the BSC with a HEPA filter, an overflow flask may be omitted.
Cleaning Spills in BSC
- Clean spills immediately with 10 % bleach or other EPA-registered disinfectant for the appropriate contact time (e.g. 10 minutes) followed by 70 % ethanol to avoid pitting of steel surfaces.
- Do not allow any potential contamination on the interior surfaces to remain as this will increase the risk of spreading potentially infectious materials beyond containment of the BSC.
- If spilled liquid enters through the front or rear grilles, close the drain valves and pour in 10 % bleach or other EPA-registered disinfectant. Allow appropriate contact time, (e.g. 10 minutes) and then drain. Follow up with a wash of 70 % ethanol into the drain pans.
- Carefully handle any paper towels or absorbent material used when cleaning the BSC as they may be sucked up into the exhaust plenum. If this occurs, the cabinet body may need to be opened by a certified BSC technologist to remove the object. This will also require that the BSC is recertified for use.
Decontamination of BSC
- Immediately following the manipulation of infectious agents in the BSC, decontaminate surfaces and BSC contents with 10 % bleach or other EPA-registered disinfectant for the appropriate contact time (e.g. 10 minutes) followed by 70 % ethanol to avoid pitting of steel surfaces. Do not allow any potential contamination to remain on the interior surfaces.
- When work is finished for the day, surface decontaminate all items that are to be brought out of the BSC prior to their removal.
- After removal of all items, the interior walls and the interior window surface of the BSC should be decontaminated with 10 % bleach or other EPA-registered disinfectant for the appropriate contact time (e.g. 10 minutes) followed by 70 % ethanol.
- Establish a routine (e.g. monthly) schedule for “deep” cleaning the BSC which would include decontaminating all interior surfaces and under the grill surface to remove any potential contamination.
- When work is completed, the cabinet should be allowed to operate for three to five minutes undisturbed to purge airborne contaminants from the work area.

References
The CDC has created a series of videos that demonstrate the Factors Affecting BSC Airflow and the Safe use of a BSC.
- Biosafety in Microbiological and Biomedical Laboratories (BMBL, 6th Ed.)
- OSHA Fact Sheet for Biosafety Cabinets
- A Guide to Biosafety & Biological Safety Cabinets by ESCO
- Heat sources in a Biosafety Cabinet Compromise Experimental and User Protection
- National Institutes of Health Design Requirements Manual, Appendix A: Biological Safety Cabinet Placement Requirements for New Buildings and Renovations
- Biosafety Cabinet (BSC) Placement Requirements for new Buildings and Renovations
- How Class II Biological Safety Cabinets Work