Background and Overview of Hazards
Piranha solutions are a mixture of concentrated sulfuric acid with hydrogen peroxide, usually in a ratio of 3:1 to 7:1. They are used to remove trace amounts of organic residues, such as photoresist, from substrates. The mixing procedure is an exothermic reaction that can reach temperatures of 100⁰C or higher. The reaction of hydrogen peroxide on concentrated sulfuric acid produces highly activated and oxidizing peroxymonosulfuric acid (H2SO5), also called Caro’s acid [1].
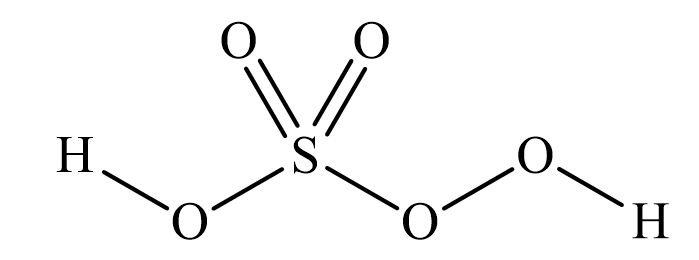
Figure 1 Peroxymonosulfuric Acid
Depending on the preparation procedure (see below), piranha solutions can contain up to 5% peroxymonosulfuric acid. This highly reactive species makes piranha an efficient solution for oxidizing organics. Like most peroxides, peroxymonosulfuric acid can be highly unstable and/or explosive depending on conditions [2]. Because piranha solutions are highly corrosive and oxidizing mixtures of sulfuric acid, peroxymonosulfuric, and hydrogen peroxide, they react violently with organic material and can cause an explosion. In addition, gas evolution can lead to pressure build-up and explosion if the solution is stored in a closed container.
Due to the described safety hazards and problems with safe disposal of waste solutions, piranha should be used only if there is no other alternative. Check if a less hazardous substitute such as KOH/ethanol, NoChromix, or Nano-strip can be used. Commercially available piranha etch solutions such as Nano-strip contain 10% peroxymonosulfuric acid and less than 1% hydrogen peroxide. These solutions are made from high purity compounds and provide a more stable, safer, and more consistent alternative to self-prepared piranha of varying quality.
Safe Handling
Due to the explosion hazard, never work with piranha when alone in the lab. All work should be conducted with secondary containment provisions in the event of spills.
Protect yourself from skin contact by always wearing Personal Protective Equipment (PPE) when preparing or handling piranha solutions:
- Appropriate laboratory attire, i.e., long pants, closed toe shoes, and a lab coat.
- Wear at a minimum disposable gloves compatible with sulfuric acid. Change them frequently and immediately after exposure. When handling large amounts (>500mL) or when splashing is more likely, wear acid resistant gloves with extended cuffs made from rubber, butyl, neoprene, or viton. Check the manufacturer’s rating for sulfuric acid. Always check the gloves for holes. Wash hands with soap and water after removing gloves.
- Safety glasses with side shields or splash goggles as eye protection are a minimum.
- A face shield, an acid resistant apron above a lab coat, or an acid smock should be worn whenever there is no barrier to prevent splashes to the face/body (e.g., when the sash is raised).
Fume Hood Use
All handling of piranha solutions must be done in a properly functioning fume hood compatible for acid use. Before you begin, lower the sash as much as possible to provide a barrier. The sash should be raised no higher than 18 inches (marked with an arrow on the test sticker) to properly capture fumes.
- Never remove an open container containing piranha from the hood.
- Clearly label the hood with a warning sign when there is piranha present.
- Do not store any organic material in the hood while piranha solutions are present.
- If the solution has to be heated, wait until it has stabilized, then use a hotplate with over-temperature protection to prevent boil-over and do NOT leave it unattended. Avoid heating piranha solutions over long periods of time (more than one hour) as it only accelerates the decomposition of the active species (see TIP under Preparation Procedure below), leading to a less effective cleaning solution.
Preparation Procedure
Prepare only as much as is needed; do NOT store any piranha solution.
Typically, 30% hydrogen peroxide in water is purchased for laboratories. Mixtures of 50% peroxide or stronger should be avoided due to the possibility of explosion [3]. If 50% hydrogen peroxide is required, vessel cooling MUST be used.
Prepare piranha solutions only in glassware or Teflon. Piranha reacts with many plastic materials. Make sure all glassware is clean and dry before using.
Mix the hydrogen peroxide solution with the sulfuric acid SLOWLY and expect the container to become hot. The sudden increase in temperature may lead to boiling or even splashing.
Any batch made over 100 mL requires cooling in an ice bath when mixed.
Tip:
Heat generated in the reaction will accelerate the decomposition of peroxymonosulfuric acid as well as that of hydrogen peroxide, resulting in a less effective cleaning solution. Previous research has shown that increased yields of peroxymonosulfuric acid can be achieved through good mixture agitation and cooling [4,5]. For best results, add cold hydrogen peroxide in portions to sulfuric acid. Reversing this order may lead to a reduced yield of peroxymonosulfuric acid [6].
Wait for the mixture to stabilize. Rinse and dry the substrate before SLOWLY immersing it. Remember that piranha solution is only for cleaning off RESIDUES.
Alternatively, the substrate can be immersed in the sulfuric acid before SLOWLY adding the hydrogen peroxide.
Warning:
NEVER add any organics to piranha solution, it could cause an explosion. Any chemical containing a C-H bond, e.g., acetone, isopropanol, ethanol, photoresist, detergents, is organic. Even small amounts of organics could make the piranha solution unstable.
Emergency Procedures
Skin Contact
Remove contaminated clothing. Flush with copious amounts of water for at least 15 minutes. If a burn is visible or if you feel pain, seek medical attention.
Eye contact
Use the eye wash to rinse eye thoroughly for at least 15 minutes, occasionally lifting upper and lower lids and rolling the eye balls. Seek medical attention after flushing the eye.
Inhalation
Move into fresh air immediately. Seek medical attention in the case of respiratory irritation, cough, or tightness in the chest. Symptoms may be delayed.
Ingestion
Do not induce vomiting. Seek medical attention immediately.
Provide the medical team with this fact sheet and the Safety Data Sheets (SDSs) for sulfuric acid and hydrogen peroxide.
Spills
An acid spill kit must be readily available before working with piranha solution. It should contain:
- Acid neutralizer such as sodium bicarbonate or calcium carbonate or other commercially available inorganic neutralizer,
- Acid resistant gloves,
- Dust pan and broom,
- Plastic container with lid or heavy duty plastic bag for collecting the neutralized debris.
Do NOT use paper towels, rags, or other organic material to absorb a spill as they may spontaneously ignite.
Spills should be neutralized immediately by covering the spilled solution with acid neutralizer. Sweep up the neutralized material with absorbent pads or a broom. Dispose of the debris as neutralized piranha waste and list the chemical you used as neutralizer.
If the spill is too big to be cleaned up safely, evacuate the area immediately, alerting others. If possible, close the door to prevent vapors from spreading. Call 911 immediately.
Storage
Store sulfuric acid in an acid cabinet. Hydrogen peroxide solutions should be stored in a refrigerator to slow decomposition. Refer to section 7 of the SDS for storage instructions.
Due to its highly reactive nature, do not store piranha solution. Mix it fresh for each application.
Disposal
If possible, neutralize spent piranha solution as you generate it. Put five times as much ice as the amount of the solution you want to neutralize into a container large enough to hold the ice, the piranha and the neutralizing solution (e.g., use 500 g of ice for 100 ml piranha solution). Pour the spent piranha solution onto the ice and then slowly add 1M sodium or potassium hydroxide solution while stirring until a neutral pH is reached.
Alternatively, if no ice is available, fill the bottom of a container (10 times the volume of the piranha solution) 1 inch high with dry sodium bicarbonate and cover it with water. Slowly pour the piranha solution in small portions onto the sodium bicarbonate. Carbon dioxide will form, and the solution can quickly foam over. Stir and wait for the gas to escape before adding more piranha solution. Make sure that solid sodium bicarbonate is left at the bottom of the container and add more if it is used up.
If the waste solution does not contain any regulated metals (arsenic, barium, cadmium, chromium, copper, lead, mercury, nickel, selenium, silver, zinc), the neutralized solution can be poured down the drain.
If you are generating large amounts, the solution contains the metals listed above, and/or the neutralization cannot be performed safely, collect the spent solution according to the following instructions. Read the instructions BEFORE preparing and using piranha and contact DRS if you have any questions or concerns.
- Do not move piranha waste from the chemical fume hood where it was generated.
- Prior to collection and storage of the piranha solution, it must be left in an open container inside a chemical fume hood in order to cool down and allow the gases from the solution to dissipate for at least 24 hours.
- The cooled piranha solution may be transferred into the piranha waste bottle. When transferring make sure no heat is produced or reactions are occurring.*It is important to remember that this used piranha cleaning solution is still concentrated sulfuric acid with an undetermined hydrogen peroxide concentration. Care must be taken not to allow the solution to be mixed with organic solvents, as this will cause a violent reaction and possibly an explosion.
- Following the transfer, the piranha waste bottle contents must be swirled to achieve agitation (see SWIRLING PROCEDURE below for more information).
- Do not place caps on piranha waste bottles. Caps will be put on by the DRS chemical waste staff at pickup.
- Do not fill piranha waste bottles above the waste fill line. (DRS waste staff will supply piranha waste bottles with fill lines beginning March 16, 2009.) Over-filled containers will not be picked up.
- Maintain a log of all additions to piranha waste bottles including the user name, date, time, amount, concentration (3:1, 5:1, etc), and verification of swirling. (See PIRANHA LOG SHEET below.)
- Do not keep piranha waste on hand for extended periods of time. The LAST ADDITION to a piranha waste bottle should be no later than 3 months after the first addition in the PIRANHA LOG SHEET.
- When generating piranha waste, additional swirling (see SWIRLING PROCEDURE below for more information) and wait time is required and must be documented on the PIRANHA LOG SHEET.
- A trained lab representative is required to sign off on the log sheet and submit it with the chemical waste pickup request. Please request the piranha waste for disposal using the chemical waste software here: https://www.drs.illinois.edu/chemicalwastepickup and email a copy of the log sheet to DRS-Waste@illinois.edu.
Swirling Procedure
Following each addition, the contents of the piranha waste bottle must be swirled to achieve agitation. Stirring with glass rods or stir bars is not recommended since scoring of the container may occur and stir bar coatings may be damaged or removed by piranha solutions. Following the LAST ADDITION – as with all other additions – the contents of the piranha waste bottle must be swirled. In addition to noting the time and date, personnel performing the agitation should take note of either heat or gas evolved after swirling under REACTION OBSERVED and COMMENTS. After a minimum of 48 hours, the contents should again be swirled and the action documented.
A piranha waste bottle may be considered CLEARED FOR SUBMISSION after the second consecutive agitation with no reaction observed. These consecutive agitations must be separated by a minimum of 48 hours.
References
[1] C.W. Jones, Applications of Hydrogen Peroxide and Derivatives, Royal Society of Chemistry, Cambridge UK, 1999.
[2] The Merck Index, 13th Ed., Whitehouse Station NJ, Merck & Co. INC., 2001.
[3] P.G. Urben (Ed.), Bretherick’s Handbook of Reactive Chemical Hazards 6th Ed., Oxford, Butterworth-Heinemann, 1999.
[4] Apparatus for generating an oxidizing reagent for the treatment of polluted water. U.S. Patent No. 3939072, Feb. 17, 1976.
[5] Process for the generation of peroxyacids. US Patent No. 5141731, Aug. 25, 1992.
[6] Peroxoacid Manufacture. International Patent No. WO 92/07791, May 14, 1992